Abstract
Background: DLBCL is highly heterogeneous in underlying biology and clinical behavior. Several high-risk disease features and poor prognostic factors are associated with a higher propensity for refractory disease or relapse after standard R-CHOP therapy; these subset patients require novel strategies to improve upon outcomes. Single-agent TAK-659, a novel oral SYK inhibitor, has demonstrated efficacy in heavily pre-treated DLBCL (Gordon et al., Clin Cancer Res, 2020). We report results of a phase I single institution, single arm dose escalation study that assessed safety of 1 st line treatment with R-CHOP and adjunctive TAK-659 for treatment naïve high-risk DLBCL.
Methods: Patients aged ≥18 years, ECOG 0-2 with untreated stage I-IV DLBCL with high-risk features defined as, ABC/non-GCB subtype, high-intermediate or high-risk NCCN-IPI (score ≥4), MYC gene rearranged by FISH including double hit lymphoma (DHL), double expressing DLBCL (DEL; overexpression of MYC ≥40% AND BCL2 ≥50% by IHC respectively), or previously treated transformed low-grade lymphoma without prior exposure to anthracycline, were eligible. Patients were treated with R-CHOP for 1 cycle on or off study followed by combined treatment with R-CHOP and TAK-659 for an additional 5 cycles on study. TAK-659 was dosed daily with dosing escalated from 60mg (dose level 1), to 80mg (dose level 2) to 100mg (dose level 3) based on a 3+3 design. The primary objective was to determine the safety and establish the maximum tolerated dose of TAK-659 when combined with R-CHOP in the front-line treatment of high-risk DLBCL. Secondary objectives were to assess preliminary efficacy of this combination as determined by overall response rate (ORR) by PET-CT (Lugano 2014 criteria), progression free survival (PFS), overall survival (OS) and establish the pharmacokinetics of TAK-659 according to dose.
Results: 12 pts were enrolled from Dec 2019 to Nov 2021. The median age was 64 yrs (range 25-75); 8 (67%) had stage III/IV disease, 4 (33%) with high risk NCCN-IPI ≥ 4. Histology included 7 (58%) with de novo DLBCL (4 GCB, 3 non-GCB subtype DLBCL) including 7 (58%) with DEL, 3 (25%) with transformed FL, 1 (8%) with Richter's and 1 (8%) with DHL.
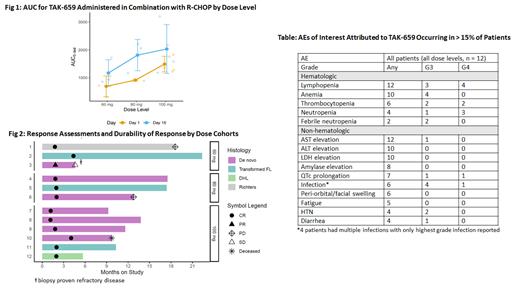
Dose level 3 (100 mg) was established as the MTD. PKs were measured pre- and post-dose D1 and D15 of cycle 2; Cuzick's test signaled an increase in AUC by dose level on D1 (p = 0.01) but not on D15 (Fig 1). ORR was 100% (CR 92%; Fig 2). With a median follow-up of 14.2 months, 1 pt had primary refractory disease (ABC and DEL), 2 pts with CR subsequently progressed (1 non-GC DLBCL, 1 Richter's) and 1 died of cardiogenic shock unrelated to study drug. The 12-month PFS and OS rates were 82% and 90% respectively with estimated 18-month PFS and OS rates of 68% and 90% respectively.
The most common treatment related adverse events (TRAEs) attributed to TAK-659 were lymphopenia (n=12, 100%), infection (6=, 50%), AST elevation (n = 12, 100%) and ALT elevation (n = 10, 83%) (Table). Incidence and severity of transaminitis was consistent with prior reports for this agent. Most common grade 3/4 toxicities were hematologic. Median number of cycles of TAK-659 delivered was 5 (range 3-5). TRAEs led to TAK-659 dose modifications in 8 (67%) pts, though none at dose level 1: 2 (17%) required permanent dose reductions (both for lung infections), while 5 (42%) required discontinuation (4 for infection, and 1 for febrile neutropenia). R-CHOP administration was delayed in 2 pts because of TAK-659 related TRAEs. Aside from dose modifications of vincristine for peripheral neuropathy, no additional dose modifications for R-CHOP were needed. Infections encountered included bacterial and opportunistic infections (1 each for PJP, CMV and aspergillosis) and 1 case of COVID. Growth factor prophylaxis and anti-fungal therapy were not mandated; PJP prophylaxis was advised for CD4 counts < 200 at initial diagnosis.
Conclusion:
TAK-659, a novel SYK inhibitor combined with R-CHOP in pts with newly diagnosed high-risk DLBCL including DLBCL transformed from follicular lymphoma and DEL produces high CR rates; survival at 12 months appears promising. A dose of 60 mg was well tolerated, did not require dose modifications and maintained a similar AUC to the MTD of 100 mg with ongoing treatment. Opportunistic infections were noted with this treatment combination suggesting that patients should receive aggressive anti-microbial prophylaxis with future evaluation of this combination.
Karmali: BeiGene: Consultancy, Speakers Bureau; Morphosys: Consultancy, Speakers Bureau; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Takeda: Research Funding; Karyopharm: Consultancy; EUSA: Consultancy; Janssen/Pharmacyclics: Consultancy; AstraZeneca: Speakers Bureau; BMS/Celgene/Juno: Consultancy, Research Funding; Genentech: Consultancy; Epizyme: Consultancy; Roche: Consultancy. Ma: Beigene: Research Funding, Speakers Bureau; Juno: Research Funding; AstraZeneca: Honoraria, Research Funding, Speakers Bureau; Loxo: Research Funding; Janssen: Research Funding, Speakers Bureau; Abbvie: Honoraria, Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Research Funding, Speakers Bureau. Winter: BMS: Other: Husband: Data and Safety Monitoring Board; Agios: Other: Husband: Consultancy; Actinium Pharma: Consultancy; Janssen: Other: Husband: Consultancy; Epizyme: Other: Husband: Data and Safety Monitoring Board; Gilead: Other: Husband: Consultancy; Ariad/Takeda: Other: Husband: Data and Safety Monitoring Board; Karyopharm (Curio Science): Honoraria; Merck: Consultancy, Honoraria, Research Funding; Novartis: Other: Husband: Consultancy, Data and Safety Monitoring Board. Gordon: Zylem Biosciences: Patents & Royalties: Patents, No royalties; Bristol Myers Squibb: Honoraria, Research Funding.
TAK-659 will be discussed for the treatment of DLBCL (not FDA approved for this indication)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal